(3) Modern and Ancient Microbialites
Microbial mats are widely distributed in modern environments despite the apparently special circumstances required for their formation. Their propensity to stabilise and modify surficial sediments, and at times, induce their lithification into laminated biosedimentary deposits, known as microbialites, is one of the few means by which microbial community activity is imprinted into the rock record. Since different communities of species interact uniquely with sedimentation and environmental factors (evaporation, desiccation, waves, currents, fluid composition, temperature), each microbialite will have an overall morphology, fabric and mineral component that is indicative of the conditions under which it formed. Crucially, morphological and compositional comparisons between modern mineralised mats with Precambrian microbialites has led to significant advances in our understanding of Earth’s early biosphere, before the onset of multicellular life. Our research group has been focusing on two different types of modern microbialites; (a) thermal spring deposits and (b) marine/lacustrine stromatolites and thrombolites, with the aim being to elucidate the mechanisms underpinning (c) ancient microbialite formation.
(a) Thermal Springs
Thermal spring deposits are typically composed of amorphous silica (sinters) or calcium carbonate (travertines), with the type of mineralisation wholly dependant upon the composition of the discharged vent fluids. Travertines tend to form in “mesothermal” springs where the bicarbonate-rich effluent have temperatures ranging from 40-75°C. Aragonite is the dominant CaCO3 polymorph at temperatures >40°C. Amorphous silica precipitates from neutral to alkaline, “hyperthermal” waters with temperatures in excess of 75°C, but depending on the state of supersaturation, silica can also precipitate at much lower temperatures. Individual thermal spring deposits are architecturally complex and show extensive lateral and vertical variations. Each biofacies may be characterised by a unique microbial assemblage that developed in response to the operative hydrodynamic, geochemical and temperature conditions. However, what remains lacking is a full understanding of how different microbial assemblages form the unique biosedimentary features (i.e., morphology, fabric) intrinsic to each deposit. My interest in hot springs began at Krisuvik, Iceland (Schultze-Lam et al., 1995; Konhauser and Ferris, 1996; Konhauser et al., 2001) where we examined in detail the columnar microstromatolites forming at temperatures between 30-40°C. Several important findings were made. First, we showed that cyanobacterial silicification contributed significantly to the overall sinter formation, with nearly 50% of the structure comprising biomineralised cells. Second, cyanobacterial silicification was rapid, as evident from the integrity and preservation of the mineralized cellular structures. Third, the microstromatolites were laminated, with the cyclicity related to seasonal variations in microbial activity. Fourth, recolonisation of the solid silica surface occurred by free-living bacteria: cell motility was not responsible for the laminations. For those cells that have become buried, they can remain viable as long as there is sufficient light for cell maintenance, but in time, they lyse and become part of the degradable organic pool utilised by the underlying chemoheterotrophic populations.
(Left Figure) Hot spring at Krisuvik, Iceland. (Center Figure) Sinter laminations, comprising silica (white) and microbial (green) layers. (Right Figure) SEM image of the silica layers (white) overlain by the microbial layers which consist of cellularly intact and vertically-aligned, filamentous cyanobacteria. From Konhauser et al. (2001).
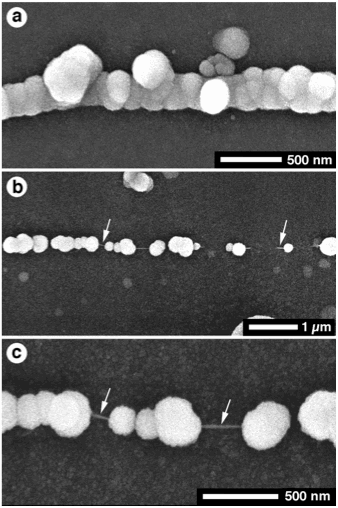
Work at the Waimangu geothermal area, North Island in New Zealand (with colleagues from the Universities of Alberta and Saskatchewan) has also shown that silicification in silica-supersaturated hot springs can be extremely rapid. After having placed glass slides for only 90 hours in shallow marginal waters of Iodine Pool (with a temperature between 70-100°C, and 440-457 ppm SiO2), we observed that the indigenous microbes appeared well preserved, with their general morphology, diameter, length, and presence/absence of septa being readily apparent. However, most of the silicified microbes lack any key features that would allow accurate comparisons with extant taxa. Moreover, the presence of collapsed filaments and pseudo-fossils complicate any efforts to relate the extant silicified cells to ancient microfossils (Jones et al., 2004).
(Left Figure) Scanning electron micrographs (SEM) of filament covered with multiple layers of opal-A spheres, found on glass slides left in Iodine Pool for 90 h. (a) General view of a silicified microbe. (b, c) Multiple layers of opal-A spheres encrusting filament. (Center Figure) SEM of pseudofilaments found on glass slides left in Iodine Pool for 90 h. (a) Small-diameter, filament-like structure. (b) Lateral continuation of the filament-like structure shown in (a). Widely spaced opal-A spheres connected by thin mucal strand (arrows) should be noted. (c) Opal-A spheres connected by mucal strand (arrows). (Right Figure) Collapsed filament c. 75% of the original diameter. From Jones et al. (2004).
A follow-up study at Frying Pan Lake also demonstrated that the dark green microbial mats, which cover the floor of the outflow channel, give rise to columns of various sizes and shapes in the shallower marginal waters. Once the columns reach the water level, the mats spread laterally to form a lilypad stromatolite. The lilypads are characterised by a raised, dark green rim, 4–5 mm high, that encircles a flat interior covered with a distinctive orange-red mat. The microbes forming the columns and lilypads are being actively silicified. The stromatolites are formed of: (i) flat-lying Phormidium filaments (P-laminae), (ii) upright filaments of Phormidium that are commonly associated with Fischerella (U-laminae), and (iii) mucus, diatoms and pyrite framboids (M-laminae). P-laminae dominates most of the columns, with tripartite cycles of P-, U-, to M-laminae being found mostly in the upper parts of the stromatolites. The transition from the P- to U-laminae is marked by a change in the growth pattern of the Phormidium and branching of Fischerella, which was probably triggered by a change in environmental conditions (Jones et al., 2005).
(Left Figures) Field photographs of stromatolites in Frying Pan Lake outflow channel. (A) General view showing stromatolites (white arrows) located along the south shore and the north-east corner of the outflow channel; Black arrow indicates flow direction. (B) Shallow water along the south shoreline of outflow channel with a green microbial mat on channel floor, isolated columns that do not reach water level (white arrows), two large lilypad stromatolites, and loose floating pieces of microbial mat along the shore. Largest lilypad stromatolite is ca 0.75 m in diameter. (C) Columns that do not reach water level. Note gas bubbles in water. (D) Lilypad stromatolite with outer, dark green raised rim surrounding flat interior that is covered by orange and light green microbial mats. Interior part of lilypad is covered by water that is up to 5 mm deep. Note gas blisters in light green mat and gas bubbles in surrounding water. The white arrow indicates flow direction. (E) Lilypad-shaped stromatolite showing orange mats in centre, dark green mat around the raised edge, scattered patches of uncovered opal-A (white), and small pieces of desiccated microbial mats resting on stromatolite surface. From Jones et al. (2005). (Right Figures) (A) SEM image of the change in growth attitude of the filamentous cyanobacterium, Phormidium sp., at the transition from P-lamina to U-lamina in a stratiform stromatolite from Ohaaki Pool, New Zealand. Arrow denotes silica precipitation. (B) Representation of the structural and fabric evolution in the same stratiform stromatolite, showing how large cavities between pillars in U-lamina fill with isopachous amorphous silica cement that is precipitated from subsurface water flowing through the porous sinter. (Modified from Konhauser et al., 2004.)
Most recently, our same group has studied the formation of travertine deposits in Lake Bogoria, the Kenya Rift valley. We demonstrated that the distribution of lacustrine carbonate stromatolites in the saline and alkaline lakes were strongly influenced by fluctuations in lake level, mostly under climate control, and by related changes in the depth of boiling (Renaut et al., 2013). During relatively arid phases meteoric recharge of groundwater is reduced, the lake is low and becomes hypersaline, and the reduced hydrostatic pressure lowers the level of boiling in the plumbing system of the hot springs. Any calcium carbonate precipitation then occurs below the land surface. By contrast, during humid phases, the dilute meteoric recharge increases, enhancing geothermal circulation, but the rising lake waters, which become relatively dilute, flood most spring vents. Much of the aqueous Ca then precipitates as lacustrine stromatolites on shallow firm substrata, including submerged older travertines. Optimal conditions for subaerial travertine precipitation in these lakes occur when the lake is at intermediate levels and may be favoured during transitions from humid to drier conditions. We refer to the vertical range of lake levels within which benthic carbonate stromatolites form on stable substrata as the "stromatolite window".
Hot springs of the Loburu delta, Kenya. (A) The interdistributary embayment, where most of the travertine hot springs are located. Bogoria Escarpment in the background. Field of view across the delta (left to right) is ca. 1.3 km. (B) Spring KL5, a typical ebullient pool spring, ca 3.5 m in diameter. Efflorescent crusts of silica (opal-A) and Na-carbonate salts surround the pool margin, but travertine is absent. (C) Spring KL15, the northernmost spring at Loburu, is a seepage spring ca 4 m in diameter without a vent pool. The spring has thin subaerial opaline silica–trona–thermonatrite crusts around the vent but no travertine. (D) Microbial mats in outflow channel network of spring KL14B, where T = 40 to 70°C. Modern mats are unmineralised except thin surficial opal-A crusts produced by wicking, and Na–HCO3–CO3 efflorescent salts on some exposed surfaces. From Renaut et al. (2012).
(b) Marine/Lacustrine Stromatolites and Thrombolites
Most of our knowledge about the mechanisms underpinning modern stromatolites (usually laminated structures) and thrombolites (macroscopically clotted structures) comes from modern marine intertidal environments. There, mat lithification typically occurs within a few centimetres of the depositional interface by the precipitation of calcium carbonate cement to form hard, current-resistant structures. Contrary to what we might predict, the actual role of cyanobacteria in microbialite formation is principally by trapping and binding processes in which the filaments are able to intertwine and incorporate detritus within their EPS to form a cohesive mat-like structure. Indeed, the cementation that takes place is, in fact, induced by the heterotrophic bacterial populations that utilise the cyanobacterial remains buried into the anoxic layers. The chemoheterotrophic processes include nitrate ammonification [reaction 1], and most importantly sulphate reduction [reaction 2]. These forms of metabolism contribute to localised increases in HCO3 levels, and eventually a state of supersaturation with respect to calcium carbonate. This, in turn, leads to the precipitation of micritic cement that trap both living and dead microorganisms in a lithified matrix, often to the ultimate demise of those entombed microorganisms.
[1] 2(CH2O) + NO3 + Ca CaCO3 + NH4 + CO2
[2] 2(CH2O) + SO4 + Ca CaCO3 + H2S + CO2 + H2O
Recently, meter-sized thrombolites, coated by well developed zonally differentiated microbial mats, have also been discovered growing in the shallow waters (depth <1 meter) of a restricted hypersaline lagoon on the Archipelago Los Roques in Venezuela. Work led by my former PhD student (Daniel Petrash) has focused on the lithification of these microbialites, which peculiarly, contain gypsum (Petrash et al., 2012). The process is thought to proceed as follows: First, extracellular polysaccharides (EPS) comprising the microbial mat concentrate Ca and other metal cations by adsorption from the hypersaline waters. Second, some of these bound metals then serve as nucleation sites for primary calcite precipitation. Third, while carbonate phases are forming in some zones of the mat, in others zones they are being re-dissolved due to the acidity generated by the metabolism of sulphide-oxidising bacteria. Fourth, as the dissolved sulphide is oxidised into sulphate, the pore-water become saturated with respect to gypsum (CaSO4). Fifth, as primary gypsum precipitates within the structures, endolithic sulphate-reducing bacteria metabolise the sulphate moiety in the mineral phase, while simultaneously oxidising the EPS trapped during accretion. Sixth, the partial dissolution of gypsum leads to increased localised alkalinity, supersaturation with respect to calcium carbonate, and ultimately pseudomorphic aragonite replacement; this differs from the calcite cement in being enriched in C and depleted in minor and trace metals initially associated with the EPS. The biogeochemical processes occurring in this thrombolite-constructing lagoon represents a novel field site for studying the chemical and isotopic processes characterising early diagenetic gypsum and the role microbes play in its precipitation, dissolution and calcification.
In another lagoon at Los Roques, we observed the interstitial occurrence of spheroidal aggregates of nanometer-scale Ca-rich dolomite rhombohedra forming within suboxic sediments associated with remnant microbial mats (Petrash et al., 2015). Multiple analytical tools, including EPMA, ICP-MS, synchrotron-based XRF and XRD, and spatially resolved XANES microanalyses, show that the dolomite-cemented interval exhibits depleted bulk iron concentrations, but is interstitially enriched in Mn and elemental sulfur (S ). Manganese occurs in several oxidation states, indicating that the dolomite-cemented interval is the locus of complex biological redox transformations characterized by coupled Mn and S cycling. The co-occurrence of S and mixed-valence Mn maintains a geochemical disequilibrium zone in which the buried organic matter is more efficiently oxidised, leading to high rates of alkalinity generation; thus conditions necessary for dolomite formation are met. This is the first study to demonstrate that microbially related processes in the Mn(IV)-reduction zone can facilitate modern dolomite formation. A follow-up study by Daniel then suggested that the bio-utilization of Fe, Mn, and sulphur for organic matter respiration sustained elevated pore-water alkalinity and pH, and allowed for the pre-compactional growth of interstitial dolomite within thin, laterally-continuous organic-rich marlstones in the mid-Cretaceous Maracaibo Platform, off the coast of Venezuela (Petrash et al., 2016a).
(Left Figures) General macroscopic features of the microbially-influenced sedimentation in Laguna Pirata, Venezuela. (A) Microbial mat coating a thrombolite, the mat exhibit distinctive layering related to the vertical array of bacterial communities. Note the masses of microcrystalline gypsum. Scale bar is 10 mm. (B) Close-up of thrombolites, notice microbial mat coating the structures; water depth 10 cm. (C) The dense coalescent growth of thrombolites, water column may vary from 15 cm to 45 cm. (Right Figure) Ernesto Pecoits and Stefan Lalonde setting up for some hard research.
Additional studies on modern microbialites are being conducted in freshwater Laguna Bacalar, Quintana Roo, Mexico. Here, my former MSc student (Set Castro-Contreras) focused on the mechanisms by which these biosedimentary structures formed, and crucially, why they were even present in freshwater. The reason this is perplexing is that it is widely believed that modern mats are generally confined to harsh environmental settings that prohibit widespread disruption and grazing by macrofaunae, such as protozoans and animals. Examples of such environments include subtidal marine environments subject to frequent sediment movement, intermittently exposed intertidal marine settings prone to periodic desiccation, hypersaline lakes and lagoons characterised by elevated salt content, hydrothermal springs where the effluent temperatures exceed the physical tolerance of most organisms, and lakes or marine basins with anoxic bottom waters. However, in Laguna Bacalar, both stromatolites and thrombolites are found growing on the south-western shore of the lake, as well as in the river feeding into the lake, and coexisting with grazing and burrowing brachiopods and bivalves (Castro-Contreras et al., 2014). Stromatolites display internal lamination, attributed to the precipitation of low Mg-calcite and the upward migration of the cyanobacteria present during periods of low sedimentation. Thrombolitic-stromatolites show internal lamination in addition to internal clotting. The clotting is seen as a result of binding and/or trapping of micritic peloids by cyanobacteria, and are attributed to periods of high sedimentation. The carbonates in both microbialites had similar C- and O- stable-isotopic signatures, both enriched in C relative to bivalves, suggesting photosynthetic CO2 uptake was the trigger for carbonate precipitation. This implies that the rate of microbialite growth is largely a function of ambient carbonate saturation state, while the texture is especially dependent on accretion rates and sediment deposition on their surface. Importantly, the coexistence of these thriving microbialites with grazing animals suggests that the latter do not significantly inhibit microbialite growth, thereby calling into question the link between the decline in microbialite abundance and diversity during the Phanerozoic and the evolution of metazoans. Varying sedimentation rates are likely important in controlling the distribution of thrombolite-stromatolite packages in the geological record, given the importance of this factor at Bacalar. This opens up the possibility that the marked increase in the abundance of thrombolites in the Phanerozoic might be linked, in part, to changes in the particulate load on carbonate platforms.
(Left Figure) The freshwater thrombolites at Laguna Bacalar. (Right Figure) The internal structure of the thrombolitic-stromatolites.
(c) Ancient Microbialite Formation
Stromatolites are particularly common features of the Precambrian shallow-marine rock record, and the similarities between the micro-textural features they contain with more recent analogues is an aspect not lost on geologists trying to understand the environment under which early microbialites formed. Unfortunately, most Precambrian stromatolites rarely contain microfossils. Therefore, evidence for microbial involvement in their accretion is cryptic, and may possibly be recorded, to a better degree, by the chemical signatures associated with both the carbonate cement and the organic and sulphur/sulphide inclusions present within their secondary mineral phases. From the study of the modern thrombolites in both Venezuela and Mexico, we have already observed the enrichment of certain bioessential trace elements, such as Co, Ni, Cr, Cu, Zn, in the microcrystalline carbonate cement that formed in association to decaying microbial mats.
The next obvious step in this research is to try and ascertain whether similar trace metal enrichments similarly exist in Precambrian stromatolites. However, measuring accurate trace element distributions in these Precambrian rocks is challenging, particularly because of the heterogeneous composition and sub-micron size of individual grains in their fabrics. Indeed, an accurate quantification of such trace metal concentrations (a few 10s of ppm) in more recent laminar carbonates has proven difficult using LA-ICP-MS due to a combination of issues, including uneven ablation of the material, non-representative sub-sampling, differences in the transport efficiencies of components to the plasma, and the lack of suitable matrix-matched standards for calibration purposes. In addition to these analytical constraints, the chemical variability due to the presence of metal reactive non-carbonate micro-inclusions, such as organic matter or sulfur species, cannot be resolved with the average beam diameter of currently operative laser ablation systems (40-60 µm). To resolve these issues, Daniel Petrash recently utilised the synchrotron facilities at both the Canadian Light Source (CLS) in Saskatoon and the Advanced Photon Source (APS) in Chicago to obtain high-resolution analyses of trace elements in several key stromatolites, including the 1.9 Ga Gunflint cherts in northern Ontario. By comparing the trace element concentrations of various stromatolite units within the Animikie Basin, our data revealed that these stromatolites reflect dissimilar burial diagenetic histories (Petrash et al., 2016b). We, therefore, concluded that the textural features and chemical signals likely reflected contrasting rates of continental runoff and solute delivery, or the temporarily and spatially variable evolution of diagenetic fluids due to mixing with exogenous fluids, rather than the vertical redox structure of shallow Precambrian seas as generally assumed in the literature.
(Left Figure) Synchrotron-based XRD results on zoned carbonate from a sample from the Frustration Bay locality. (A, B) Zoned carbonate crystal (BSEM + EDS). (C, D) Fine-scale microXRD analyses of such crystals revealed that they are silicified Fe-rich members of the dolomite series, with ankeritic cores and Fe-dolomite cortices. (Right Figure) Micro-XRF elemental maps. The panels show a representative laminar zone of a stromatolite from the Frustration Bay microfossiliferous locality of the Gunflint Formation (sample is FB74g). The colour-coding convention assigns blue to the minimum and red to the maximum deconvoluted peak area of the element. From Petrash et al (2016).
Modern mats can also inform on the cycling of major elements or the production of biogenic gases, such as oxygen. In this regard, we have surveyed the in situ production rate of modern cyanobacterial mats across a diverse range of environments to assess what their O2-production potential is today, and crucially, what it may have been in the Precambrian. This is important because a remarkably coherent ensemble of evidence points to a significant accumulation of atmospheric oxygen for the first time in Earth’s history beginning ca. 2.45 Ga, the so-called Great Oxidation Event (GOE). Briefly, this includes the disappearance of detrital pyrite, uranitite and siderite from fluvial and deltaic deposits, an increase in the retention of iron in paleosols, an enrichment of Cr and U in banded iron formations, and perhaps most importantly, the disappearance of sedimentary sulphur isotope mass-independent (S-MIF) anomalies indicative of atmospheric SO2 processing in the absence of appreciable ozone. However, several trace element and isotopic proxies have recently suggested oxidative weathering hundreds of millions of years earlier. The superposition of pre-GOE signals for oxidative weathering at a time of global anoxia represents a conundrum for which the most accepted explanation is that pre-GOE oxidative weathering is the result of transient oxygenation events driven by "oxygen oase" in the marine realm. Lalonde and Konhauser (2015) recently proposed an alternative model, that being intense O2 generation – and immediate consumption – at sub-mm scales by benthic oxygenic photosynthesis in the terrestrial realm. Despite the absence of a UV-protective ozone layer in the Archean, a terrestrial phototrophic biosphere may have existed in various sheltered environments, including biological soil crusts and freshwater microbial mats covering riverbed, lacustrine, and estuarine sediments. We calculated that the rate of O2 production via oxygenic photosynthesis in these ecosystems provides sufficient oxidising potential to mobilise sulphate and a number of redox-sensitive trace metals from land to the oceans while the atmosphere itself remained anoxic with its attendant S-MIF signature.
(a) Comparison of annual fluxes of principal O2 sources and sinks in the pre- and post-GOE Earth system (lower green box) with the size of major O2 reservoirs pertaining to the GOE itself (upper blue box). All values are in Tmol or Tmol/y O2 equivalents. This diagrammatically indicates the magnitude of the disconnect between the annual O2 fluxes associated with surficial redox processes (including modern oxidative continental weathering) and the amount of O2 equivalents implicated in the GOE itself. (b) Close up of A depicting annual O2 source and sink fluxes, in terms of O2 equivalents, contributing to Earth’s surface redox balance. Assuming the median diurnal benthic O2 production rate (0.16 nmol cm−2 s−1), the potential net O2 production rate by terrestrial microbial ecosystems is displayed as a function of percent modern continental surface coverage as a blue dashed line (blue area encompasses 90% of compiled rates). Only small degrees of benthic photosynthetic coverage are required to account for the earliest signals of oxidative weathering, highlighting a strong but previously unrecognized potential sensitivity in these signals to the evolution of terrestrial oxygenic photosynthesis. It is important to note that these signals may be generated with no net redox imbalance and thus independent of atmospheric oxygenation. From Lalonde and Konhauser (2015).
The goals for the future are to better understand the biogeochemical processes that lead to the accretion of both siliceous and calcareous microbialites. By comparing Precambrian stromatolites with similar biosedimentary features in the modern, we may finally be able to extrapolate their primary community structure from those few preserved microfossil assemblages. This will provide valuable insights into the microbial diversity existing at that time, and hence, the evolution of early life. Major questions being sought are:
-
What causes the lamination in some microbialites, and how do the microbes respond to diurnal and seasonal fluctuations?
-
What mineralogical, textural and geochemical changes occur during diagenesis?
-
How does the pore-water and gas composition within overlying microbial mats influence microbialite mineralization?
-
What caused ancient stromatolites to become dolomitised, and why is dolomite so rare today?
-
Can the trace element distribution be used as "biosignatures” for Precambrian microbialites?
-
Can Archean mats be deciphered for their potential O2-production potential?
-
-
2-
2+
2+
+
2+
-
2-
+
2+
-
2+
12
2+
O
O
-






A
B
C
D








