(1) Surface Reactivity of Biomass
The surface layers of microbes facilitate adsorption and mineral nucleation reactions by providing chemically reactive sites in the form of organic ligands. Surface complexation models that employ microbial ligand concentrations and proton-ligand stability constants are used to describe reactions at the cell-water interface. The main motivation for these studies is to better understand the mechanisms by which microbes might adhere to solid substrata, influence the transport of metal contaminants in the subsurface, and ultimately to design effective bioremediation strategies. Because surface complexation models are based on thermodynamic parameters, they can be applied to predict biotic reactions taking place in geochemically diverse systems. However, whether or not different microbes show different surface chemical reactivities, or whether a single microbial species exerts control over the adsorptivity of its surfaces, remains poorly understood. In this regard, our research group has over the past few years conducted a number of experiments and field-based studies under various environmental conditions aimed at measuring changes in cell surface reactivity, using either (a) natural microbial mats, (b) intact cells, (c) extracellular polymers (EPS), or (d) specific components of the EPS, and (e) most recently, biochar, a carbon-rich solid produced via pyrolysis of biomass.
(a) Natural Microbial Mats
Led by my former PhD student (Stefan Lalonde, now a CNRS Researcher at the European Institute for Marine Studies in Brest), we applied cell surface characterisation techniques to natural microbial populations at hot springs in Yellowstone National Park, USA. In the first study, we evaluated the concentrations and thermodynamic properties of surface ligands associated with microbial mats and hydrous ferric oxides (HFO), the concentrations of metals that they bound, and the chemical composition of the fluids in which they were bathed. Consideration of all three parameters has allowed, for the first time ever, a direct comparison between organic and inorganic surface chemical reactivity and metal sorptive capacity in the same natural setting. We showed that microbial and HFO surfaces have similar concentrations of surface ligands that are available to serve as sorption and mineral nucleation sites, yet those sites differ in their affinity for specific elements as a function of their chemical composition (Lalonde et al., 2007a). In a second study, we collected a number of microbial mat samples from a single spring, with the goal being to ascertain differences in cell surface reactivity on small spatial scales. We showed significant variability in surface chemical reactivity between microbial communities on scales of just a few millimetres; much of that variability was due to the ratio of cells to EPS. We also demonstrated that in these mats, the presence of authigenic carbonate phases (that formed during photosynthetically-generated alkalinisation) intermixed with the EPS played a greater role in metal partitioning than the organic components of the mat ecosystem (Lalonde et al., 2007b).
Replicating environmental growth conditions and/or environmental fluctuations in a laboratory setting presents many practical obstacles. Potential changes in the reactivity of field samples during transport between the sampling site and laboratory could complicate replication. Recently, we sought to highlight the potential disparity in field conditions relative to laboratory cultures, and ultimately, to improve the application of quantitative metal adsorption predictions to more accurately replicate environmentally relevant conditions. In this regard, my current MSc student (Tyler Warchola) recently conducted fieldwork at the Fairmont Hot Springs in southeastern British Columbia, Canada, in the hope of being able to distinguish the differences in titrating microbial mats associated with travertines in the field versus in the laboratory. He demonstrated that the carbonates yielded a "carbonate spike” in the titration data introduced by the contribution of inorganic carbonate mineral dissolution and subsequent carbonate speciation changes during the transition from low to high pH (Warchola et al., 2017). This inhibited the determination of microbial surface ligand variety and concentrations. Determination of the reactivity of the mat biomass could ultimately be achieved by physically removing larger grains of authigenic carbonates, and repeatedly treating the biomass with weak acid washes until remaining carbonate solids were removed (Flynn et al., 2017).
(Left Plate) (A) Map of sampling locations in Norris Geyser Basin, Yellowstone National Park. (B) Overview of Beowulf Spring, with close up of (C) ‘yellow mat’ [labelled 1 in (B)] consisting of thermophilic, filamentous bacteria and elemental sulphur precipitates and (D) the sharp transition zone between the hydrous ferric oxide (HFO) (label 2) and the green mat of Cyanidia algae (label 3). (E) Gap spring, where terraced HFO mats accumulate with the outflow surrounding the vent. (F) Perpetual Spouter, similarly showing HFO deposition within the outflow channel. From Lalonde et al. (2007a). (Right Plate) (A) Map of sampling locations in Sentinel Meadows, Yellowstone National Park. (B) All samples were obtained from an area ~2.5 m2 (highlighted by the white box) that lay ~8 meters from the spring source. The relative position of samples representing four mat morphologies are indicated on (C): (1) subaqueous samples with vertical spire morphology (shown enlarged in (D)) were collected from a small pooled section of stream, (2) samples with streamer morphology were collected from the pool outflow channel (upper part of photograph (E)), (3) yellow mat was collected from the left side of the same outflow channel (E), and (4) white mat were collected from the central portion of the large mat bounding the proximal edge of the stream pool (C). Upon return to the laboratory, thick (~4 cm) samples of traditional mat morphology (3,4) were sectioned into upper and lower portions for individual analyses (F). From Lalonde et al. (2007b).
(b) Intact Cyanobacteria
In a series of related studies, we employed potentiometric titrations to evaluate variations in bacterial surface organic functional group chemistry using live cells of the cyanobacterium Anabaena sp. The cells were grown under a variety of batch culture conditions, and by various assimilatory nitrogen metabolisms. We observed that ligand concentrations and acidity constants were influenced by the form of nitrogen on which the cultures grew, as well as their stage of growth (Lalonde et al., 2008a). Subsequently, we grew the cells in solutions of varying ionic strength and silica concentrations and additionally showed that the cells altered their surface chemistry as a response to growth in silica supersaturated solutions (Lalonde et al., 2008b). Collectively, these two studies highlighted the complexity of microbe-environmental interactions in terms of cell reactivity, demonstrating that a single bacterial species may alter its surface chemical properties in response to environmental stimuli.
(Left Figure) Confidence intervals in multiple pair-wise comparisons of ligand concentrations plotted as a function of comparison type: between metabolism exponential phase comparisons (left), between-metabolism stationary phase comparisons (centre), and between growth phase metabolism comparisons (right). From Lalonde et al. (2008a). (Right Figure) Confidence intervals in multiple pairwise comparisons of ligand concentration plotted as a function of comparison type: between metabolism comparisons grouped as a function of Si concentration (left) and between-Si concentration comparisons grouped as a function of nitrogen metabolism (right). From Lalonde et al. (2008b).
In other studies, we focused on how microbial surface chemistry and their charge characteristics may influence the microorganism’s hydrophobic and hydrophilic properties, which in turn, affects the microbe’s ability to adhere to solid surfaces, bind metal cations and form extracellular biominerals. In this regard, my former PhD student (Vernon Phoenix, now a Professor at the University of Strathclyde) showed that the cell surface reactivity of Calothrix sp. strain KC97 could be described as a two-layered system composed of a highly anionic cell wall enclosed within a neutrally charged sheath. The two-layered distribution of reactive sites on Calothrix sp. has several important ecophysiological implications, one of them being that the cyanobacterial sheath provides a protective mechanism against detrimental biomineralisation (Phoenix et al., 2002). In the case of silicification in hot springs, this exopolymer was demonstrated to act as a filter against colloidal silica by restricting mineral precipitation onto the sheath’s outer surface and preventing detrimental silicification of the cell wall. Moreover, because Calothrix sp. strain KC97 is a benthic cyanobacterium that inhabits biofilms covering the silica sinters at hot springs, the ability of this microorganism to adhere to a substratum is fundamental to its survival in this environment because free-floating forms would be rapidly removed from the hot spring site by the relatively fast-flowing discharge waters.
Not surprisingly, many studies have suggested that bacterial adhesion is proportional to the microorganism’s hydrophobicity and inversely proportional to the bacterium’s surface charge. Along these lines, other cyanobacteria have the means to become more hydrophilic in nature, particularly when they are growing planktonically. Recent work by a former PhD student (Yuxia Liu, now a PDF at Peking University) showed that the anionic surface of Synechococcus sp. PCC 7002 allows this plankton a greater ability to bind metal cations from seawater (Liu et al., 2015). If one considers that an active bloom of Synechococcus sp. PCC 7002 may contain 10E-10E5 cells/ml, then at the calculated binding of 10E-8 to 10E-7 moles of metal per litre, these cells can play a significant role in the transfer of metals from the photic zone to the seafloor sediment upon cell lysis. In fact, this study highlights the potential that marine plankton, in general, may play in trace metal sorption in the oceans, and ultimately, their burial into marine sediments. More recently, (Liu et al., 2016) used potentiometric titrations and Fourier Transform Infrared (FT-IR) spectrometry to profile the key metabolic changes and surface chemical responses of the same Synechococcus strain during different growth regimes. This included testing various nitrogen (N) to phosphorous (P) ratios (both nitrogen and phosphorous dependent), nitrogen sources (nitrate, ammonium and urea) and growth stages (exponential, stationary, and death phase). Comparisons between the titration data (for the cell surface) and FT-IR spectra (for the average cellular changes) generally indicate (1) that the nitrogen source is a greater determinant of ligand concentration than growth phase, and (2) that phosphorus limitation has a greater impact on Synechococcus cellular and extracellular properties than does nitrogen limitation. Taken together, these techniques indicate that nutritional quality during cell growth can noticeably influence the expression of cell surface ligands and their measurable densities.
(Left Plates) (A) Samples of Synechococcus sp. PCC 7002 liquid cultures in A+ media, grown under constant illumination at 30°C, and shown here at various densities, from 12×10E7 cells/ml (far left) to 0.9×10E7 cells/ml (far right). (B) SEM micrograph of Synechococcus sp. PCC 7002 cells fixed with 2.5% glutaraldehyde, 2% paraformaldehyde and gold coated. (C) TEM micrograph of ultrathin sections showing the ultrastructure of a representative Synechococcus sp. PCC 7002 cell taken at late exponential phase. (D) TEM micrograph showing a close up of the cell wall, emphasising the lack of a sheath. From Liu et al.(2015). (Right Figure) Cd sorption edges from experiments of Synechococcus sp. PCC 7002 cells exposed to Cd concentration of 1 ppm (A), 10 ppm (B), and 25 ppm (C) over a range of pH. The biomass concentration was approximately 10 g wet weight / L. From Liu et al. (2015).
(c) Harvested EPS
In a study led by a former PhD student (Matthew Baker), he demonstrated how cold-adapted bacteria modify their surface reactivity under different pH and temperature conditions. Bacteria with EPS were shown to be more anionic than the cell wall itself because they possessed additional carboxyl groups (COOH) with a pKa (5.1 to 5.8). In turn, this increased reactivity fostered greater metal adsorption (Baker et al., 2010). Importantly, since the bacterium has the means of generating different amounts of EPS depending on environmental conditions, we speculated that the cell ultimately has the means to alter its surface reactivity to compensate for periods of either low or high solute concentrations.
(d) Alginate
Several studies have previously examined metal adsorption to bacteria, algae and fungi, with and without EPS, and they rather consistently show that removal of EPS can reduce metal binding capacity. However, due to spatial heterogeneities that arise during differentiation or stratification of regions within a single biofilm, the presence of authigenic and/or detrital mineral components, and the difficulties associated with isolating individual biofilm components (cells, EPS, minerals) in natural samples, the importance of individual EPS components towards overall EPS chemical reactivity is poorly understood. Accordingly, in a study led by my former PhD student (Daniel Petrash, now a PDF at Charles University in Prague), we focused on the biopolymer alginate in an effort to better understand the geochemical role of a major polysaccharide constituent in biofilms. Alginate is a straight-chain, hydrophilic, polyuronic acid extensively secreted by brown algae and several species of bacteria, including Pseudomonas and Azotobacter sp. Using surface complex modelling, we demonstrated that the surface reactivity of alginate is dominated by the deprotonation of carboxyl groups at pH 4; at high pH values, the alginate becomes increasingly anionic. Accordingly, alginate was able to adsorb significant amounts of Cd from circum-neutral pH solutions. In fact, Cd-carboxyl complexation constants were higher than most bacterial and algal cell walls (Petrash et al., 2011a).
(e) Biochar
Following on from the microbial work, we have also taken a more applied route by investigating the surface charge properties of biochar, the carbonaceous remains of biomass after its thermal decomposition in the absence of oxygen (pyrolysis). Biochar is a carbon-rich solid produced through the carbonization and/or pyrolysis of biomass derived from a variety of feedstocks, including straw, wood, and organic industrial wastes. Biochar is a cost-effective sorbent owing to its low production cost and its ability to efficiently remove metals and organics from water. In a study by my recent PhD student (Samrat Alam, now a PDF at Toronto) ), we used biochar (BC), petroleum coke (PC) and admixture of BC and PC to remove organic acids (OAs) from aqueous solution (Alam et al., 2016). Oil sands process-affected water (OSPW), generated on site during upgrading processes, produces tailings materials that contain high concentrations of polycyclic aromatic hydrocarbons (PAHs), and other organic acids (OAs). Organics in OSPW are also persistent pollutants, and have been found in groundwater and surface waters taken near to oil sands tailings ponds, and treating OAs from OSPW has been a major challenge. Following characterizing BC and PC to determine their morphologies, composition and functional group concentrations, we performed batch studies to assess the uptake of lauric acid (LA) and 1-methylcyclohexenecarboxylic acid (MCA) by BC, PC, and BC+PC. BC, produced in Alberta near the Athabasca oil sand mine region, showed more promise as a sorbent for both model organic acids, although PC removes significant OAs from solution and is available on-site. Admixtures of BC and PC showed synergetic effects, and seemed to be highly effective to remove organic acids from solution. The release of Ni and V from PC generally brings into question the suitability of PC as a sorbent to remove OAs and metals from OSPW. By contrast, BC does not leach heavy metals at concentrations of concern and thus, may be a more suitable sorbent.
(Left Figure) Adsorption data for lauric acid onto (A) BC at 20 g l-1, (B) BC at 10 gl-1, (C) PC at 20 g l-1, (D) PC at 10 g l-1, and (E, F) a 1:1 mixture by mass of BC and PC at 20 g l-1 and 10 g l-1, respectively (Alam et al., 2016). (Right Figure) Adsorption data for MCA onto (A) BC at 20 g l-1, (B) BC at 10 g l-1, (C) PC at 20 g l-1, (D) PC at 10 g l-1, and (E, F) a 1:1 mixture by mass of BC and PC at 20 g l-1 and 10 g l-1, respectively. From Alam et al. (2016).
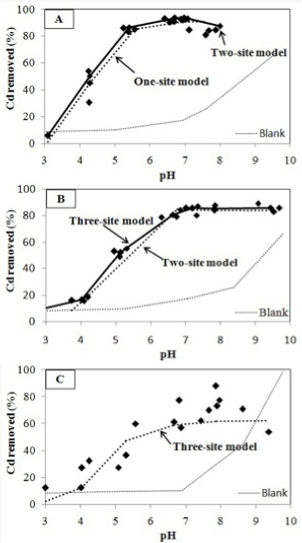
Biochar has also emerged as an amendment to release nutrients into the agricultural soil to increase crop productivity and as a sorbent to remediate metals and organics contamination. Since soils have heterogeneous physical properties across a given crop field or over a growing season, it is imperative to select the most appropriate biochar for the intended purpose and in defining the amendment level. In a recent study (Alam et al., 2018a), we investigated the adsorption of Cd(II) and Se(VI) to two agricultural soils amended with a wood pin chip biochar (WPC). The proton reactivity of each sorbent was determined by potentiometric titration, and single-metal, single-sorbent experiments were conducted as a function of pH. The resulting data were modelled using a non-electrostatic surface complexation modelling approach to determine the proton and metal binding constants and surface functional group concentrations of each soil and WPC. The SCM approach is a considerable advance over empirical modelling approaches because SCM models can account for changes in pH, ionic strength, temperature, and metal-to-sorbent ratio that may happen over the course of a growing season. Our modelling results showed that overall the SCM approach was successful in predicting metal distribution in multi-component mixtures.
While numerous studies have investigated metal uptake from solution by biochar, few of these have developed a mechanistic understanding of the adsorption reactions that occur at the biochar surface. In a series of related studies (Alam et al., 2018b; Alam et al., 2018c), we employed a combined modeling and spectroscopic approaches to describe the molecular level adsorption of divalent cations (Ni(II) and Zn(II)) and radionuclide (U(VI)) to biochar using different types of biochar produced from three feedstocks, wheat straw, wood pin chip, and sewage sludge, produced at three temperatures: 300°C, 500°C and 700°C. We applied two thermodynamic approaches; (i) surface complexation modelling (SCM) and (ii) isothermal titration calorimetry (ITC), supported by synchrotron-based X-ray absorption spectroscopy (XAS) to develop a predictive and mechanistic model of metal binding to biochar. Following thorough characterization, potentiometric titrations were carried out to measure the proton (H+) reactivity of each biochar, and the data was used to develop protonation models. SCM supported by XAS were then used to develop predictive models of Ni(II), Zn(II) and U(VI) binding to biochar, as well as provide insights into the molecular scale metal-biochar surface reactions. SCM combined with isothermal titration calorimetry (ITC) data was then used to determine the thermodynamic driving forces of metal adsorption. Our results showed that the reactivity of biochar towards Ni(II) and Zn(II) directly relates to the site densities (mole sites/m2) of biochar (Alam et al., 2018b), and U (VI) uptake is strongly influenced by U(VI) aqueous speciation at different pH (Alam et al., 2018c). Fourier transform infrared spectroscopy (FT-IR) studies confirmed the presence of proton-active carboxyl (–COOH) and hydroxyl (–OH) functional groups on the biochar surface, and coupled with synchrotron-based extended X-ray absorption fine structure (EXAFS) analyses, suggested that Ni(II), Zn(II) and U(VI) adsorption occurred at these sites. The SCM approach was able to predict the Ni(II), Zn(II) and U(VI) adsorption behaviour across a wide range of pH, in the presence of solution Ca2+, and at varying initial U(VI) and biochar concentrations. SCM combined with ITC analyses revealed that the enthalpies of protonation are exothermic and Ni(II) and Zn(II) complexes with biochar surface are slightly exothermic to slightly endothermic depending on the protonation state of biochar surface functional groups to which the metals complex. The results obtained from these combined approaches contribute to the better understanding of molecular-scale metal adsorption onto the biochar surface and will facilitate the further development of thermodynamics-based, predictive approaches to biochar removal of metals from contaminated water.
(Left Figure) Adsorption of Ni and Zn onto WS, WPC, SSBC 300°C, SSBC 500°C and SSBC 700°C, showing the 2-site best-fit models: (A) 17 µM N, (B) 170µM Ni, (C) 17 µM Zn, and (D) 170µM Zn. The open symbols represent experimental data and solid lines represent best-fit models. (Right Figure) EXAFS signals weighted by k3 spectra and R space curve fitting results for Ni and Zn adsorbed to WS. (A) k3 spectra of Ni, (B) R space of Ni, (C) k3 spectra of Zn, and (D) R space of Zn; (1) 170 µM Ni; (2) 85 µM Ni; (3) 17 µM Ni; (4) 170 µM Zn; (5) 85 µM Ni and (6) 17 µM Zn. From Alam et al. (2018a).
Synchrotron-XRF map of U(VI) distribution onto WS biochar at pH (A) 5, (B) 7, and (C) 10.
Biochar can donate, accept and transfer electrons either biotic or abiotic pathways in their surrounding environments. In a study, cost-effective, environmental friendly and robust magnetite (Fe3O4) nanoparticles (MNPs) and biochar (GW) composites (MNP-GW) were synthesized for high-performance Cr(VI) removal from aqueous solution at acidic to neutral pH ranges. Batch adsorption and reduction studies show that MNP-GW highly enhanced both adsorption and reduction of Cr(VI) from aqueous solution at acidic to neutral pH ranges compared to MNPs and GW alone, suggesting a strong synergetic cooperation between Fe3O4 and GW (Alam et al., 2018d). The Cr(VI) removal processes occurred through surface adsorption, chemical adsorption and intraparticle diffusion followed by a redox reaction. Synchrotron-based X-ray absorption spectroscopy (XAS) analysis shows that Cr(VI) reduced to Cr(III) at the surface of MNP-GW. Extended X-ray absorption fine structure (EXAFS) fitting results confirm the formation of Cr(III) precipitates as Cr(OH)3 and chromite (Cr2FeO4) nanoparticles on the surface of MNP-GW. The results demonstrate that MNP-GW has great potential for reductive immobilization and detoxification of Cr(VI) in aqueous media.
HR-TEM images of (A,B) MNPs; (C) MNP-GW (high Fe concentration); (D,E) MNP-GW (low Fe concentration); and (F) SAED pattern of MNP-GW.
In another study, a modified Community Bureau of Reference (CBR) sequential extraction method was tested to assess the composition of untreated pyrogenic carbon (biochar) and oil sands petroleum coke. The applied sequential extraction method represents a suitable technique to recover metals from these materials, and is a valuable tool in understanding the metal retaining and leaching capability of various biochar types and carbonaceous petroleum coke samples, which can be used to help assess the metals leaching potential of the materials (von Gunten et al., 2017).
Sequential extraction results showing the contributions of fractions 1-4 to the total metals concentrations. The digestion results are shown above each graph. AqRe; aqua regia. Tot: total digestion with HF. F1: exchangeable/acid soluble fraction. F2: reducible fraction. F3: oxidizable fraction. F4: residual fraction. The error bars represent standard deviations (n = 3). PW = Pinewood biochar; OW = Oak wood biochar; BW = Bamboo wood biochar and SPC = oil sands fluid petroleum coke sample. From von Gunten et al. (2017).
The goals for the future are to continue focusing on the environmental factors that influence the surface chemical reactivity of microbes, biominerals and biochar in order to improve surface complexation models designed for predicting trace metal distributions in natural settings and for the design of improved bioremediation and biorecovery strategies. Major questions being sought are:
-
How do microbial consortia bind solutes from solution, and can this work lead to the development of more applicable surface complexation models to predict reactions in natural environments?
-
How do benthic versus planktonic microbes vary their surface charge and hydrophobic/hydrophilic properties with changing environmental conditions?
-
What genetic control do bacteria have over their surface charge properties?
-
How do trace metals partition between biomass and authigenic minerals in natural systems, and do their KD values differ from experiments with isolated microbes and mineral phases?
-
How do microbes attach to solid surfaces as a means for initiating weathering reactions and soil formation?
-
How does the presence of inorganic components in biochar, such as alkali metals, clays and minerals, effect the biochar reactivity towards various potential sorbates?
-
How do complex organic ligands, such as fulvic and humic acids, impact metal adsorption by biochar since most natural and engineered systems will contain such organic ligands?